The CHIASMA Project has published a report outlining the development and validation strategy for both in vitro and computational New Approach Methods (NAMs), focussing on compliance with OECD guidelines to ensure regulatory readiness. The ‘Report on NAMs’ Requirements and Validation Strategy’ demonstrates the necessary procedures to prepare the NAMs under development in CHIASMA for regulatory submission.
The Project integrates biological and computational approaches, aiming for a combinatorial framework that enhances mechanistic understanding and safety assessment efficiency. The structured validation process, including intralaboratory and inter-laboratory evaluations, aims to produce reliable, reproducible, and ethically sound NAMs, setting new standards in toxicological testing and safety evaluation.
Overview – New Approach Methods
The 3R principles of reduction, replacement, and refinement, emphasise minimising animal use in experiments by adopting alternative in vitro models (1). Modern toxicology is increasingly focused on employing models that elucidate the mechanisms through which chemicals, drugs, materials and substances affect measurable readouts and phenotypes in biological systems. To achieve accurate mechanistic material safety assessments, it is imperative to develop and utilise relevant biological test systems. New Approach Methods (NAMs), which include in silico (computer-based), in chemico (chemical-based), in vitro (cell culture-based), and ex vivo (tissue-based) techniques, are promoted by regulatory bodies globally (OECD, ECHA, FDA and more), as effective alternatives to animal testing. These methods provide essential risk assessment data for chemicals and materials while significantly reducing the reliance on animal experimentation (2,3).
When developed according to the rigorous validation guidelines established by the OECD, NAMs offer the advantage of consistent and reproducible endpoints, which contrasts with the variable outcomes often associated with animal experiments (4). Furthermore, NAMs enhance resource efficiency, allowing for high-throughput experimentation that generates ample data for robust analysis within shorter timeframes, and algorithmic methods (in silico, in chemico) are only limited by computational requirements and the availability of curated FAIR (Findable, Accessible, Interoperable and Reusable) data. Effective mechanistic assessments from biological systems necessitate testing multiple concentrations and exposure times, increasing sample numbers and the throughput required. NAMs are well-suited for such demanding experimental designs due to their resource efficiency, and coupled with computational methods that can bring information from the wealth of publicly available data, NAMs are highly applicable for safety assessment. As a result, NAMs are becoming the foundation of contemporary toxicology and safety assessments, despite ongoing challenges to their universal acceptance and implementation (3,5). The adoption of NAMs marks a significant shift towards more ethical and efficient testing methodologies, paving the way for advancements in chemical safety evaluation without the extensive use of animals.
New Approach Methods in CHIASMA
The final goal of CHIASMA is to develop combinatorial NAMs, consisting of in vitro, omics and in silico components, which have each been developed in an iterative fashion. From the outset, in silico and omics methods will use publicly available data based on the exposure substances selected in WP1 – Project Context & Specifications. The in vitro biological NAMs will be exposed to the chemicals defined in WP1, and data from these experiments, including omics data under WP3 – In silico Methods & Models Development, will be used to fill and data gaps in the development of the in silico and omics NAMs approaches. In turn, the combined data and developed methods will be brought together to refine these combinatorial NAMs (Figure 1). Each of these stages requires an understanding of, and compliance with, the necessary regulatory guidelines for each of the methodologies employed, with an eventual goal to have regulatory ready NAMs over the course of the CHIASMA Project that can be submitted to the OECD.
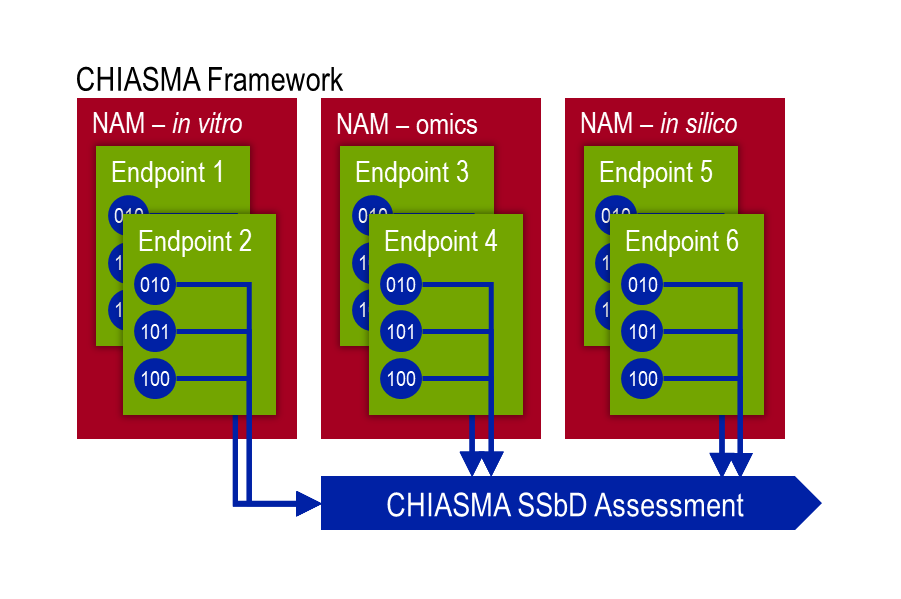
CHIASMA’s in vitro NAMs
The report discusses the following OECD Guidance Document (OECD TGs):
- OECD GD 211- Guidance Document for Describing Non-Guideline in vitro Test Methods,
- OECD GD 286 – Guidance Document on Good In Vitro Method Practices (GIVIMP), and
- OECD GD 34 – Guidance Document on the Validation and International Acceptance of New or Updated Test Methods for Hazard Assessment.
CHIASMA computational NAMs
Computational modelling and in silico approaches are beginning to become a crucial component in modern toxicology and safety assessment NAMs. Such computational NAMs involve using computer algorithms and simulations to predict the toxicity and safety of substances based on their chemical structure and known biological data. In silico models can rapidly analyse large libraries of data, to predict the potential risks of test substances, thereby reducing the need for extensive in vitro or in vivo experiments (6).
Follow this link to download the full CHIASMA Report on NAMs’ Requirements and Validation Strategy.
- Tannenbaum J, Bennett BT. Russell and Burch’s 3Rs Then and Now: The Need for Clarity in Definition and Purpose. J Am Assoc Lab Anim Sci JAALAS. 2015 Mar;54(2):120–32.
- Directive 2010/63/EU of the European Parliament and of the Council of 22 September 2010 on the protection of animals used for scientific purposes [Internet]. [cited 2024 Jun 28]. Available from: https://eur-lex.europa.eu/eli/dir/2010/63/2019-06-26
- Stucki AO, Barton-Maclaren TS, Bhuller Y, Henriquez JE, Henry TR, Hirn C, et al. Use of new approach methodologies (NAMs) to meet regulatory requirements for the assessment of industrial chemicals and pesticides for effects on human health. Front Toxicol. 2022 Sep 1;4:964553.
- OECD GD 34: Guidance document on the validation and international acceptance of new or updated test methods for hazard assessment. OECD Publishing; 2005.
- Sewell F, Alexander-White C, Brescia S, Currie RA, Roberts R, Roper C, et al. New approach methodologies (NAMs): identifying and overcoming hurdles to accelerated adoption. Toxicol Res. 2024 Mar 25;13(2):tfae044.
- Ram RN, Gadaleta D, Allen TEH. The role of ‘big data’ and ‘in silico’ New Approach Methodologies (NAMs) in ending animal use – A commentary on progress. Comput Toxicol. 2022 Aug 1;23:100232.